Regulatory Affairs Pharma and Biotech
In pharma and biotech, quality assurance and regulatory affairs can hardly be dissociated from each other. That’s why the QbD Group offers you the total package.
Not only do we take care of quality management, but we also support you throughout the entire drug regulatory lifecycle.
Whether this concerns traditional pharmaceuticals, generics, vaccines, biologicals, biosimilars, or Advanced Therapy Medicinal Products (ATMP).

Expertise overview of our RA services

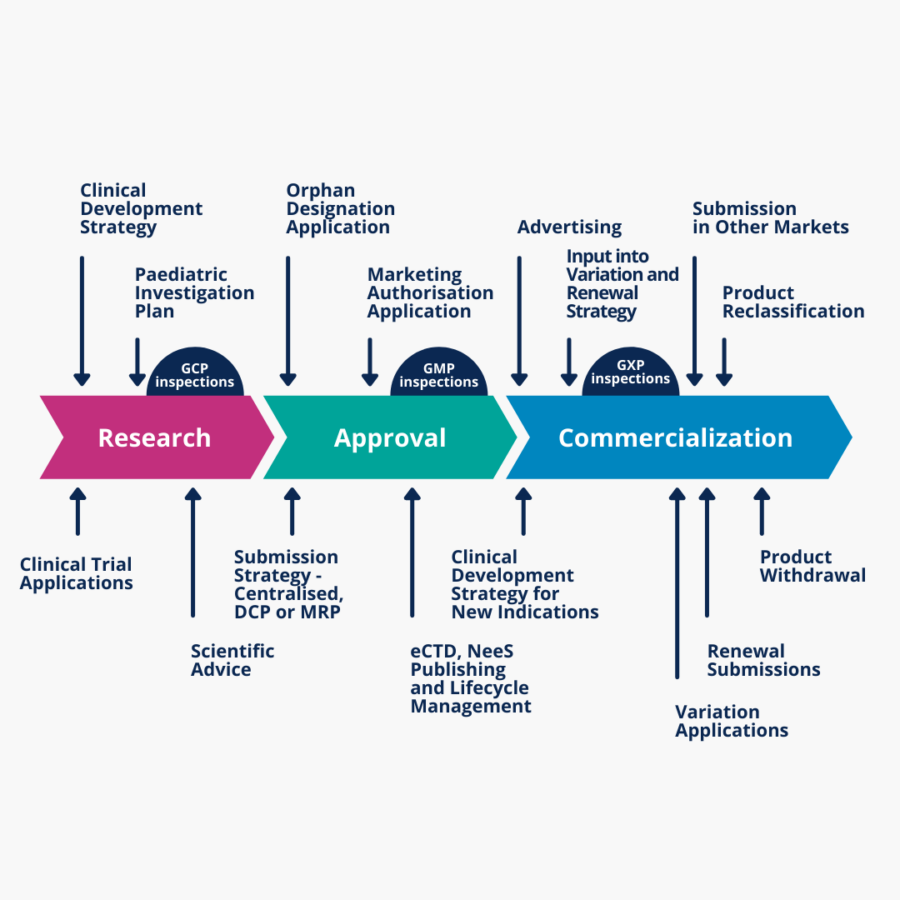
Regulatory strategy
- Clinical Study applications (CTA, IB, IMPD, etc.)
- Early development activities (PIP, ODD, PRIME, etc.)
- Regulatory submissions (MAA, eCTD, NeeS, etc.)
- Program & project management lead (PML)
- Filing/registration strategy - accelerated assessment
- Legislation interpretation
- Dossier gap analysis & due diligence
- Authority fees calculations
- Scientific advice & Protocol Assistance
Regulatory and scientific writing
- Drafting submission files (eCTD)
- Policy 0070 implementation guidance
- Enter data in EudraCT (Europe) and clinicaltrials.gov (FDA)
- Expertise with innovative ATMPs, biologicals and biosimilars
- Environmental assessment report (ERA) (Module 1.6)
- EU-RMP (Module 1.8.2), PSUR and DSUR writing
- Authoring of Non-Clinical and Clinical expert reports (Modules 2.4 – 2.5 – 2.6)
- Gap analysis and update of CMC
- CMC writing of Module 3 & Module 2 Quality Overall Summary
- Technical writing: transferring raw data into regulatory compliant reports
- Authoring or review of EU Product Information (Summary of product characteristics, labelling and package leaflet)
Global submissions
- Europe: Centralized Procedure and Decentralized, Mutual Recognition and National Procedures
- UK/GB: National Applications and Reliance/Recognition routes
- FDA projects (IND, eNDA, eBLA, ANDA)
- Experience with emerging markets
- EDQM: submission of CEP dossier
- Translations
- Readability testing
Post-Authorisation Regulatory Affairs for Pharma and Biotech
- Post-approval safety submissions (PSURs, RMP updates)
- Renewals & variations, MA transfers
- Resubmissions of MA
- Annual product review
- Article 57 database maintenance
- Review of Educational and Promotional Materials
Pharmaceutical Drugs
FLYER
Regulatory Affairs for Pharma and Biotech
In this free flyer, you will learn more about the regulatory services QbD Group provides for the pharmaceutical and biotechnology industries.
Pharmacovigilance & Drug Safety
The QbD Group provides strategic and operational support for partial or full outsourcing of Pharmacovigilance (PV) responsibilities.
Why QbD Group?
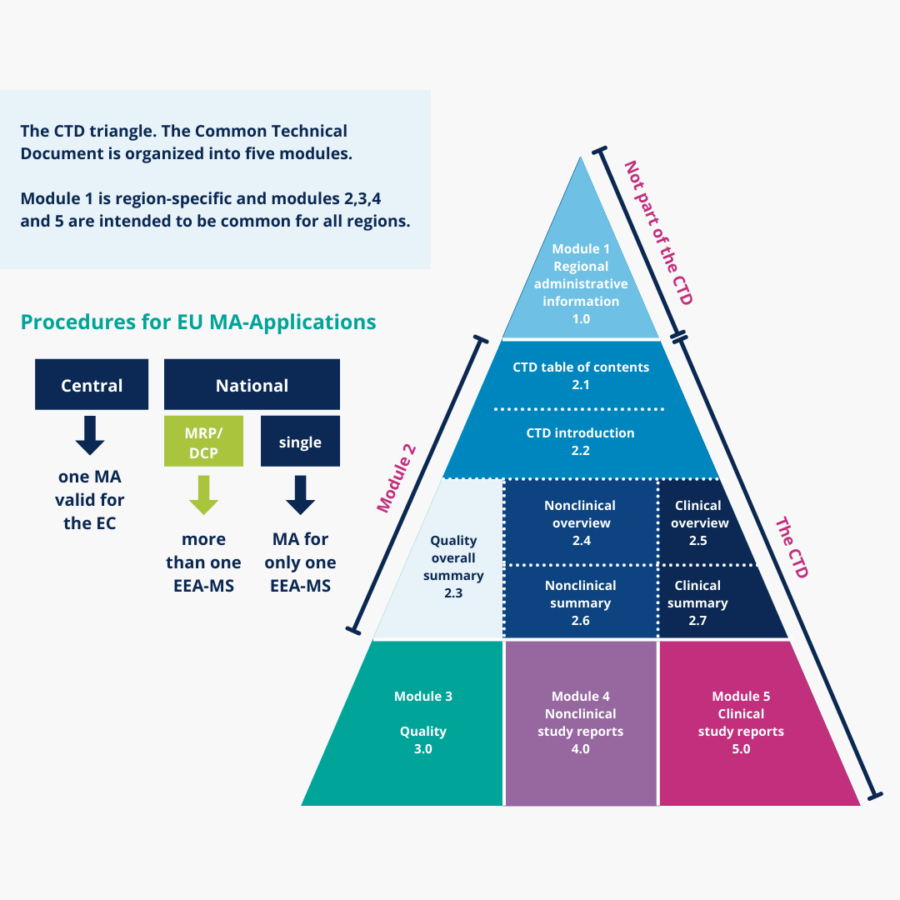
In order to transform the development, formulation and manufacturing data of active substances and/or finished dosage forms into a compliant dossier that can be submitted to regulatory authorities for review and approval, a group of expert CMC writers is needed. QbD can support you in writing the appropriate quality module in CTD based on the phase of your medicinal product, whether it’s in a clinical phase or in the marketing authorization application phase. Our experts can perform the necessary literature reviews and prepare the relevant expert reports.
We support you in the submission of marketing authorization applications for medicinal products with new or known active ingredients, with or without innovation (generics). For innovative medicines in particular, the approval procedures can be complex and time-consuming. Here, our regulatory affairs team can take the lead in preparing meetings with the authorities (scientific advice, pre-submission, clarification, application for an ATMP or orphan classification).
Our regulatory affairs officers will help you:
- develop the right submission strategy (EU: CP, NP, MRP, or DCP)
- compile a compliant eCTD dossier
- submit the marketing authorization application in the required countries, according to the predefined registration procedure, including the preparation of answers to official questions and the follow up until the end of the procedure
Once marketing authorizations are approved, they must be continuously maintained and updated to ensure that the approved manufacturing process in the dossier matches the reality at the manufacturing site(s).
Our RA team can support you in bringing your medicinal products to market and maintaining marketing authorizations throughout the life cycle. Post-approval activities (such as renewals, variations, annual product reviews, Article 57 database, transfers, etc.) can be handled by our regulatory affairs team.
A complete Regulatory Affairs solution
Our consultants offer comprehensive knowledge and expertise when it comes to Regulatory Affairs for pharma and biotech. We have the knowledge and expertise to support your team or to insource full regulatory affairs projects – all in an efficient and pragmatic way.
Our Regulatory Affairs services for pharma and biotech include writing and structuralizing registration files according to the legislation of various countries and integrating all required (technical) data.
Contact us
Contact us for more information or request a free, no-obligation proposal.