Regulatory Affairs for Medical Devices
Quality assurance and regulatory affairs can hardly be dissociated from each other. That’s why Quality by Design offers you the full package.
Not only do we take care of quality management, but we also support you throughout the entire medical device regulatory lifecycles.
Overview of our RA services

Regulatory strategy
- Clinical investigation, clinical evaluation
- Medical device classification
- Contact with Competent authorities (FDA, Notified Body, AFMPS/FAGG, …)
- Regulatory submission
- Program and project management
Global submissions
- Europe: CE marking, local registration
- Enter data in EudaMed
- FDA 510k, PMA,…
- Experience with emerging markets
Post Market regulatory
- PMS/PMCF
- Update of CER
- Renewals & Variation
- Annual report for EudaMed
Medical Devices
In-country Representative Services
Do you require representative services for your medical devices and/or IVDs? QbD’s Qarad is an independent partner that can act as EC-REP, CH-REP, or UKRP.
Person Responsible for Regulatory Compliance (PRRC)
Are you looking for a Person Responsible for Regulatory Compliance (PRRC) for your medical devices and/or IVDs?
Legal Representative Clinical Trials
Are you a sponsor with offices outside the EEA and wish to conduct a clinical trial within the EEA? QbD Clinical can act as your legal representative.
Why QbD?
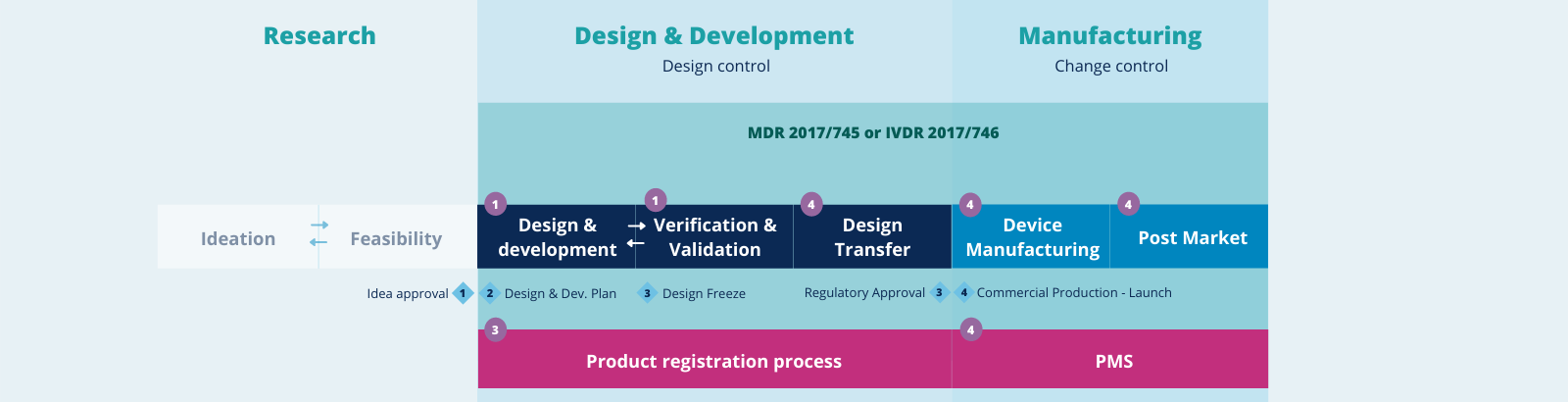
We cover all stages of the medical device lifecycle, from idea to commercialization.
1
Product Development
services
- Design control & documentation structure
- Design Verification and Validation
- Risk management
- Build supplier and partner network
- Quality Management System implementation
2
Regulatory affairs & clinical solutions
- MDR regulatory pathway
- IVDR regulatory pathway
- Technical documentation filling support
- Qualification & classification of SW as a Medical Device
- Clinical solutions
- Data management statistics
- Project & site management
- Regulatory CA, IRB/EC
- Safety, medical monitoring
- Compliance & medical writing
3
Product Registration Process
- RA strategy
- Contact NB and/or competent authorities
- Compilation of the technical documentation
4
Design Transfer & Manufacturing
- Production support
- QA support
- Quality event management
- Inspections and audits
- Post-market surveillance (PMS)
- Post market clinical data
- Technical documentation
ISO 13485:2016
- Audits - audit preparation
- QMS implementation
- eQMS solution: Scilife
Training
- ISO 13485:2016
- Medical Devices & IVDs: roadmap to CE-marking
- Medical Device Software (MDSW)
A complete Regulatory Affairs solution
Our consultants offer comprehensive knowledge and expertise when it comes to regulatory affairs. We have the knowledge and expertise to support your team or to insource full regulatory affairs projects – all in an efficient and pragmatic way.
Our Regulatory Affairs services for medical devices include writing and structuralizing registration files according to the legislation of various countries and integrating all required (technical) data.
Contact us
Contact us for more information or request a free, no-obligation proposal.