Industries
QbD supports life sciences companies throughout the entire product lifecycle, from idea to patient. Read more about our core industries below.
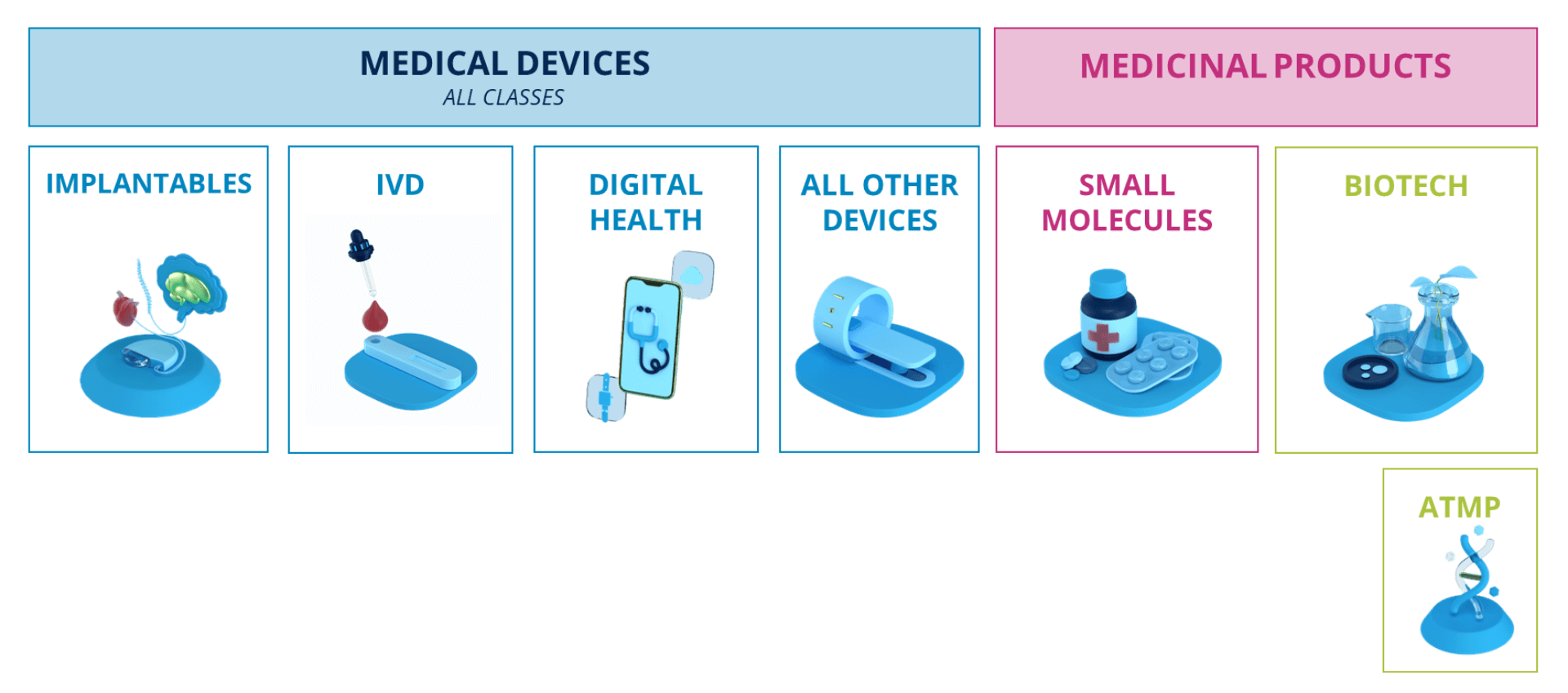
Sector overview
Medical Devices
Let’s get your medical device to market. We support you from concept to launch in the full lifecycle.
In Vitro Diagnostics
Thanks to our wealth of experience in the field, QbD is your ideal partner for all your IVD RA/QA concerns.
Digital Health
QbD can be your partner in identifying, selecting, integrating, and implementing digital technologies in healthcare.
ATMP
QbD is your dedicated partner to make Advanced Therapy Medicinal Products more stable, robust, and upscalable.
Biotech
QbD works for many biotech companies and understands that it requires a very specific approach.
Small molecule & generics
QbD offers companies specialized knowledge, expertise and software for the small molecule & generics industry.
Why QBD?
QbD supports companies worldwide in the life sciences throughout the entire product lifecycle, from idea to patient.
With more than 450 quality experts, QbD is your partner for advice and support.
+10 years experience
Full cycle support
Global presence
Best Managed Company