In the field of regenerative medicine, advanced therapy medicinal products (ATMPs) represent the next generation of medicines. It is, however, not so straightforward to translate this new expertise into a GMP process. In this article, you’ll learn more about the challenges and crucial aspects.
- genetic therapy medicinal products (GTMPs),
- cell-based therapy medicinal products (CTMPs),
- tissue-engineered products (TEPs),
- or a combination of the above.
They represent the most promising, sometimes unique, therapeutic option for various diseases in which traditional medicine has proven ineffective.
A therapy based on ATMPs is often used for the treatment of rare diseases. It is thus targeted to a patient-specific population.
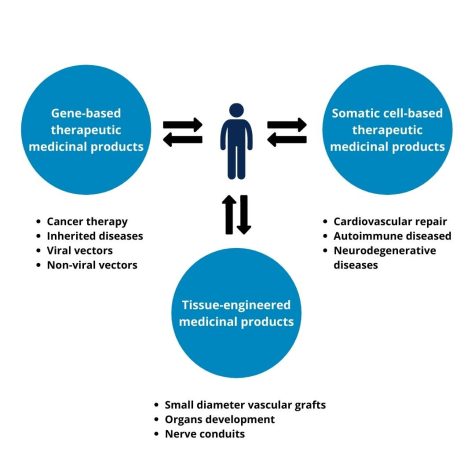
Figure 1 – Schematic representation of advanced medicinal therapeutic products. CAR: chimeric antigen receptor. Adapted from Goula et al.
Preparing ATMPs: dealing with a heterogeneous product pool
The first step in the development of ATMPs is isolating the cells or tissue from human sources. This is one of the reasons why ATMP manufacturing is considered to be strictly related to the clinical, transplantation area. Upon manipulation of the raw material, ATMPs are prepared for autologous (same individual) or allogenic (different donor) use.
- Manipulated as living cells, CTMPs are collected via apheresis, are then modified, expanded, and administrated to the patient to treat diseases or injuries at the cellular level.
- GTMPs consist of recombinant nucleic acid, intended for the regulation of genetic sequences directly in the patient (in-vivo) or in its cells after collection (ex–vivo).
- TEPs are based on the use of immature (e.g. stem cell cells) or differentiated stem cell populations, with the purpose of repairing structurally compromised tissues in the patient.
Given the nature of ATMPs, they represent an extremely heterogeneous pool of products whose preparation differs substantially from mainstream biopharmaceutical manufacturing. Therefore,
- a high degree of technical expertise,
- strong clinical background,
- and very specialized production processes
are required for the development of ATMPs. As a consequence, the history of developing ATMPs has been rather bumpy until recently.
From ATMP to GMP: a bumpy history
Lack of legislation and poor harmonization
For a long time, the major contribution to the development of ATMPs came from hospitals, small companies, and academic institutions. Facilities that often have restricted financial resources and limited competencies to navigate the required regulatory procedures necessary to address the GMP industrial standards.
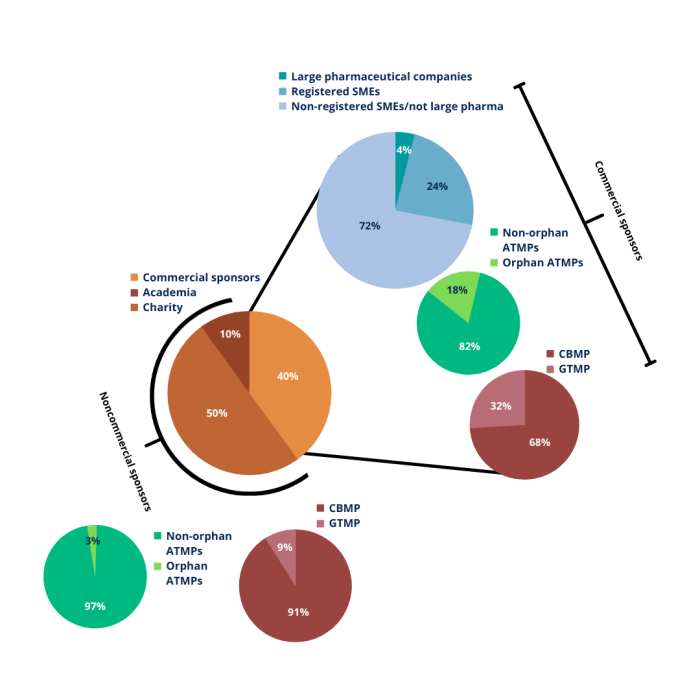
Figure 2 – The ATMP landscape in Europe 2004–2010. Adopted from Maciulaitis et al
For years, lack of an actual legislative framework characterized ATMP development, which led to a variety of individual academic/hospital manufacturing settings.
On top of that, the poor harmonization in GMP application within the EU’s member states, that was in effect until 2009, exacerbated the issue. Nevertheless, as pharmaceutical products, ATMPs have to reach manufacturing and quality standards. This is a complicated matter when translating the ATMP’s development into a GMP process.
Difficult implementation and application
The second criticism encountered when transferring the manufacture of ATMPs to the Pharmaceutical Industry concerns the implementation of the abovementioned GMP standards: given the unique characteristics and the high patient-specificity nature of the ATMPs, the common GMP standards cannot simply be applied. Therefore, despite the fact that the development of ATMPs plays a key role in the treatment of rare or degenerative diseases, bringing these medical products to market is not an easy process.
CAT and centralized procedures: a great leap forward
A major step forward in the regulation of ATMPs was taken in January 2009, with the establishment of the Committee for Advanced Therapies (CAT), at the European Medicines Agency (EMA).
This multidisciplinary scientific committee aims at reviewing the quality, safety and efficacy of ATMPs according to standards established by regulatory authorities. Today, along with the introduction of centralized procedures for the manufacture of ATMPs, the CAT helps pharmaceutical companies establish ATMP production. Click here to learn more about the main hurdles of marketing an ATMP.
Crucial aspects of manufacturing ATMPs in a modern GMP environment
Despite the bumpy history, ATMPs development is an active, ongoing process. However, translating the manufacture of these therapeutic products into a GMP environment still poses significant challenges.
The intrinsic variability of the starting material as well as of the finished product requires manufacturing procedures that necessarily deviate from those of traditional biologic products.
The extreme diversity of the starting material obtained from a patient is due to – among other things – that person’s age, health and illness. This requires impeccable batch identity and tracking. Moreover, ATMP therapies require a complex trial design, as a unique patient population is targeted. Therefore, unlike common biopharmaceuticals, we need flexible, well-monitored and controlled processes to cope with the complexity of ATMPs.
Despite the introduction of centralized procedures for the manufacture of ATMPs, ATMPs developers are asking for more specific guidance regarding the GMP requirements that govern the manufacturing process.
Transport from patient to site of manipulation
To begin with, one of the first obstacles to the production of ATMPs under GMP conditions mentioned in the literature is the transportation of the collected sample from the patient to the site of manipulation. The context of the intraoperative environment somehow escapes GMP regulation, which contradicts the need to avoid contamination. A possible solution to this problem could be operating in a closed system, but the very definition of a closed environment is controversial.
Sterility of the final product
Another reported case where producers of ATMPs have to deal with the lack of unified GMP requirements is that of sterility. As with CTMPs, the presence of whole live cells prevents filtration through sterilization-grade filters, so the final product necessarily cannot be sterilized. Since final sterilization cannot be performed, the sterility of the final product must be ensured through routine sterility testing during manufacture and aseptic process simulations.
Logistics related to ATMPs manufacturing
The logistics associated with the production of ATMPs are also very critical, as the stability of the human sample and of the final ATMPs decreases significantly with the duration of transportation and is strictly temperature dependent. These and many more aspects of the production of ATMPs must be strictly regulated to meet GMP standards.
Clean rooms, risk-based approach and IPCs
As challenging as it may be, developers of ATMPs have powerful tools to support the manufacturing process in a GMP compliant manner. The installation of clean rooms and the application of a risk-based approach through the method of failure modes and effects analysis (FMEA) can ensure the safe disposal of processed tissues and cells at every step of the production of ATMPs. In-process controls (IPCs) are also an essential tool to control the safety and quality of the entire production process.
To establish process development according to the QbD approach, QbD offers software to structure process optimization according to the quality by design paradigm, from setting up the CQA, and thorough risk assessment to experiment design and analysis in a single package. Click here to read more.
Monitoring quality of ATMPs: open challenges for ATMPs developers
Gaps in legislation
Nowadays, regulating the quality of ATMPs is a hot topic for health authorities. The use of products of human origin is notoriously connected with the transmission of infectious diseases and adverse reactions.
Therefore, quality and safety must be warranted during every stage of the manipulation of the processed tissues and cells. Because of the unique characteristics and great heterogeneity of ATMPs, in terms of quality assessment, current regulatory gaps must be filled.
For instance, in the analyses of the raw starting material for the assessment of identity and purity of a cell population, are difficult to be performed under a standardized conditions.
Evaluation of potency or mechanism of action
The same applies to the assessment of efficacy or mechanism of action. One of the reasons underlying the discrepancy between GMP requirements and the nature of ATMPs is the large volume of cell product required for quality testing, as opposed to the scarcity of material or limited batch size, particularly for autologous and personalized therapies.
Quality assessment tests must also comply with GMP standards, which means minimizing operator-related variability in favor of standardized, reproducible processes, emphasizing the need for automation of the manufacturing process and its monitoring system. Immunofluorescence analyses based on the use of specific markers represent a solid alternative to microscopy for the assessment of cell identity, for example.
The importance of a registered Qualified Person
It is clear that in order to successfully convert the production of ATMPs into a process according to GMP guidelines, adjustments must be made at several levels. To support quality control and meet the needs of developers of ATMPs regarding the application of GMP requirements, a registered qualified person (QP) can certify the quality and safety of the final product prior to release.
Download our CbD Whitepaper
- Goula et al. doi:10.14740/jocmr3964
- R. Dream BioPharm International-11-01-2018 Volume 31, Issue 11 Pages: 30–34
- R. Maciulaitis et al. doi:10.1038/mt.2012.13
- Regulation EC No1394/2007 EUR-Lex – 32007R1394 – EN – EUR-Lex (europa.eu)
- Regulation EC No 726/2004 EUR-Lex – 32004R0726 – EN – EUR-Lex (europa.eu)
- F. H. Fritsch et al. doi: 10.1159/000447645
- EU directive 2001/83/EC CL2001L0083EN0110010.0001.3bi_cp 1..1 (europa.eu)
