Additive manufacturing, particularly 3D printing, has attracted strong attention of the medical devices and medical community at large over the past decade.
This bottom-up production technology uses raw material to create complex, three-dimensional objects. This relatively new technology has the potential to provide flexible and patient-specific solutions for medical device manufacturing.
3D printing is currently used for multiple applications such as:
- Surgical implants (e.g. protheses, stents, …)
- Tumor, organ and anatomical visualization for operation planning
- Surgical equipment
- Tissue engineering
- In vitro diagnostic medical devices (IVDs)
This innovative fabrication method also brings new challenges to guarantee quality and patient safety. This blog introduces you to Joe DiMeo and the state-of-the-art applications of 3D printing that changed his life. The medical world still has a lot of challenges to tackle and 3D printing could be one of the solutions.
3D printing medical devices: what are the benefits?
3D printing medical devices can bring many benefits to both patients and medical professionals. 3D printing technology makes it possible to manufacture bottom-up involved designs with a high degree of complexity that are otherwise unfeasible.
Moreover, the uncomplicated production of customized and personalized medical products, drugs, and medical devices with a single 3D printer is one of the most important advantages in the medical field.

Figure 1 – The benefits of 3D printing for the medical and medical device industries
But it doesn’t stop there! Cost-effectiveness, increased productivity and speed, the democratization of design and manufacturing, rapid prototyping, low material waste, and improved collaboration are also benefits associated with 3D printing in medical devices and in medicine.
Improving the quality of patient care is the ultimate goal. If 3D printing technology is properly implemented and fully utilized, it will have a beneficial impact on the lives of many people. Highly complex surgeries involving multiple conditions simultaneously can be more thoroughly prepared and completed in a shorter time frame. A good example of this will be discussed in detail.
Case study: face and double hand transplant
In 2018, Joe DiMeo was in a terrible car accident, after which the 22-year-old spent more than 3 months in a coma. He suffered such severe injuries to both his hands and face that a normal life was no longer possible, and unfortunately, traditional medical treatments would fall short.
That’s why a Belgian company, Materialise, teamed up with NYU Langone surgeons for the world’s first full face and double hand transplant. This highly complex and time-sensitive surgical procedure was facilitated by 3D printing, virtual 3D design, and meticulous procedure planning.
In the months before surgery, a 3D model of the patient’s face and arms was created, allowing for virtual planning and visualization of various outcomes. After 14 months of preparation, a team of about 80 healthcare providers was ready to begin the 23-hour surgery.
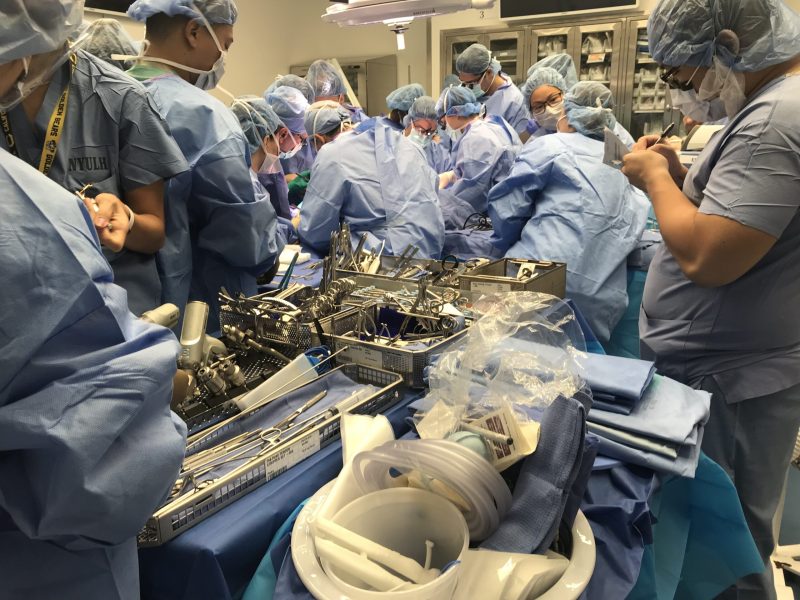
Figure 2 – Rehearsing Joe DiMeo’s surgery in a lab environment to develop and fine-tune the surgical plan
(Source: Materialise)
During this surgery, custom 3D-printed surgical guides were used to cut, drill and place medical implants. The patient-specific guides fit perfectly on Joe’s bones and ensured that surgical procedures were performed without the need for revision. This is due to the high accuracy and precision of the 3D-printed parts.
This well-prepared, patient-specific and coordinated effort led to a successful reconstruction. The custom-designed guidance systems for bone repositioning and fixation were critical during the surgery to achieve high precision and reduce overall surgery time. Joe’s function, appearance, and overall quality of life improved dramatically, bringing the use of 3D printing in medicine into the spotlight.
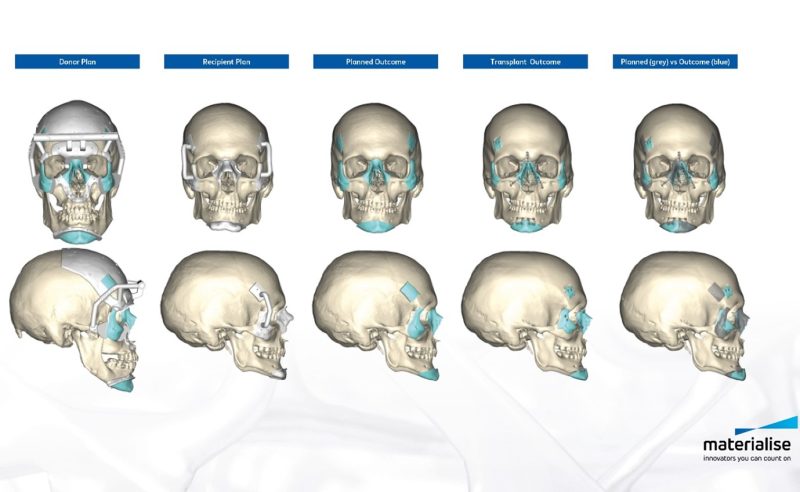
Figure 3 – Pre-surgical planning allowed surgeons to virtually select and place medical implants to predict the optimal fit (Source: Materialise)
Guaranteeing quality: the need for regulation when 3D printing medical devices
As these types of new manufacturing technologies emerge, regulations must keep their finger on the pulse to continue to provide safe and effective care to patients.
Many 3D printing applications fall under the current European Medical Device Regulation (MDR) 2017/745 or are in the transition from directives (MDD) to regulations (MDR). The MDR has made the effort to include 3D printed medical devices compared to the MDD. Although there are still some pitfalls, progress is being made.
Conducting the necessary conformity assessment procedures, compiling a technical file, preparing the EU Declaration of Conformity, and affixing the CE mark, if applicable, are necessary requirements before 3D printed medical devices or appliances can be placed on the market.
3D printers are subject to mandatory EU standards. The health, safety, and performance of the printer must be guaranteed. Harmonized specific technical solutions are included in the MDR or a different technical solution may be chosen, which requires a detailed explanation of compliance with EU requirements.
Great strides are being made, but we still have a long way to go!
3D printing is already in widespread use to create medical devices, but there are still many challenges ahead. The classification of these devices is not always straightforward, which can make their validation difficult, resulting in the difficulty of ensuring quality for each patient.
QbD has experts ready to guide you through this process. Don’t hesitate to contact us should you have any questions about your medical device and/or its regulatory framework.