In the post-COVID pharmaceutical landscape, electronic records and signatures are indispensable, guided by the 21 CFR Part 11 compliance framework. This blog post delves into the significance of data integrity in pharma and elucidates 21 CFR Part 11, and its objectives. Plus, get your hands on our free 21 CFR Part 11 compliance checklist and questionnaire available for download below.
FREE PDF
21 CFR part 11 compliance checklist and questionnaire
Download our free 21 CFR Part 11 compliance checklist and questionnaire to make your automated systems compliant.
The importance of data integrity in pharma
If you have worked in the pharmaceutical industry for some time, you’ve probably realized at some point that protecting data from accidental or intentional alteration, distortion, or deletion is the key to maintaining reliable health records that comply with established regulations.
Data integrity refers to maintaining complete and intact data records in their original data context in relation to other data records. In this sense, you achieve data integrity when your data records are valid to provide necessary services and comply with the regulations that drive your organization.
21 CFR contains rules for data integrity. Its Part 11 has everything to do with electronic data and electronic signatures (more on that below). Implementing data integrity improves the reliability of documentation.
Protecting data from accidental or intentional alteration, distortion, or deletion is the key to maintaining reliable health records that comply with established regulations.
What is 21 CFR?
The Code of Federal Regulations or “CFR” is the codification of the general and permanent rules published in the Federal Register by the executive departments and agencies of the federal government of the United States of America.
It is divided into 50 titles representing broad areas to which federal regulations apply. Title 21, in this case, handles “Food and Drugs“.
Each title is divided into chapters, which usually bear the name of the issuing agency. Each chapter is further divided into sections dealing with specific areas of regulation.
Source: eCFR
What is 21 CFR Part 11?
CFR Title 21 focuses on “Food and Drugs“, and Part 11 is devoted to “Electronic Records” and “Electronic Signatures“.
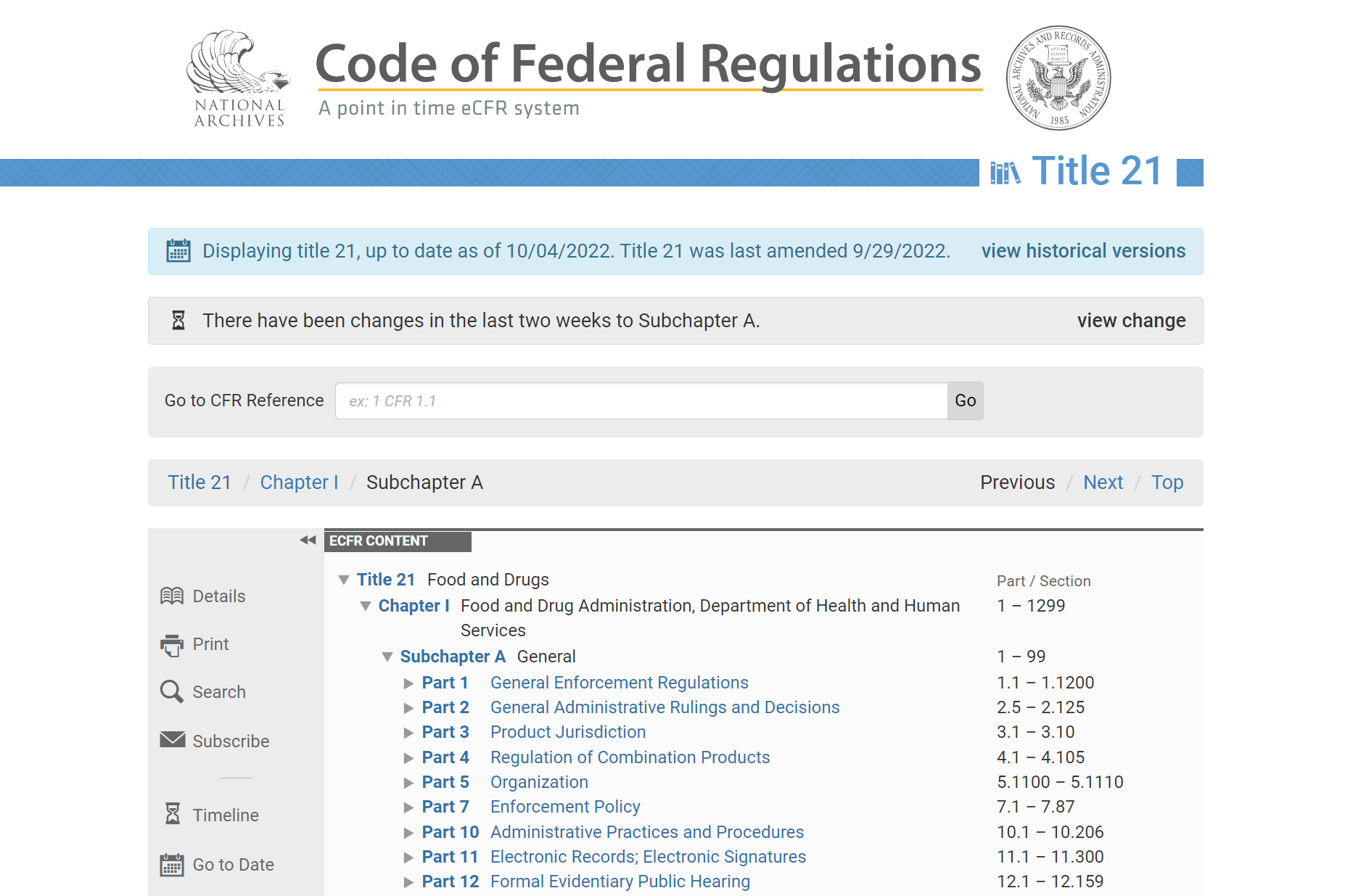
Source: eCFR
In particular, 21 CFR Part 11 regulates how electronic records and electronic signatures should be applied in data management in the pharmaceutical industry.
Therefore, data integrity under 21 CFR Part 11 relates to how these regulations guide the management of electronic records and electronic signatures.
Example
For example, systems that generate electronic records must have controls that ensure authenticity, integrity, and reliability.
Among other things, this rule is there to ensure that signed records cannot be altered by users and that the system can determine when a record was created, altered, or deleted by a user of the system.
In the case of electronic signatures, these controls should include:
- the name of the singing user,
- the day/time the signature was executed,
- and the meaning of the signature.
So “Revision”, “Approval”, and “Author”, for example.
Moreover, two separate identification components should be used, namely, an identifier and a password, which should only be used by the true owner.
Organizations and companies in the pharmaceutical industry are required by 21 CFR Part 11 to implement good business practices by clarifying the methods by which electronic records and signatures are presumed to be accurate, valid, reliable, confidential and equivalent to paper records and handwritten signatures.
What is the goal of 21 CFR Part 11?
Promotion of data integrity
As noted above, the primary purpose of 21 CFR part 11 is to promote data integrity in the use of electronic records and signatures to ensure that records are reliable, complete, and comprehensive.
In other words, pharma professionals must adhere to these standards to increase the reliability of electronic records and signatures. In this way, the storage, processing, retrieval, and dissemination of data/information become transparent and reliable to users.
Maintaining the relationship between data records
In addition, these standards ensure that the relationship between data records is maintained. Maintaining the data relationship is important because it allows you to retrieve information from different sources to improve decision-making.
In fact, 21 CFR Part 11 improves data integrity by ensuring that retained data is reliable and equivalent to the data in records and paper signatures.
21 CFR Part 11 compliance checklist
Indeed, one of the main advantages of computer systems for the pharmaceutical industry is that they allow us to maintain reliable, up-to-date electronic records that are easier to process, store and retrieve.
Advances in information technology have made health records management very easy, but again, of course, the regulatory framework must be followed to act with integrity.
Want to check if your systems are compliant? Download our free 21 CFR Part 11 compliance checklist and questionnaire here:
FREE PDF
21 CFR part 11 compliance checklist and questionnaire
Download our free 21 CFR Part 11 compliance checklist and questionnaire to make your automated systems compliant.
Need help to comply with 21 CFR Part 11?
At the Qbd Group, we offer consulting and services to enable the use of electronic records and signatures in your systems, as well as all documentation and security and functionality testing to meet the requirements of 21 CFR Part 11.
Need help with 21 CFR part 11 compliance and data integrity? Don’t hesitate to contact us.

