QbD Module - The efficient EBR system for ATMPs
Advanced Therapy Medicinal Products (ATMPs) offer great new opportunities for the treatment of patients and their diseases or injuries. In ATMP laboratories and cleanrooms all over the world, experts work on the manufacturing and quality control of these medicines, based on genes, tissues or cells. Efficient management of workflows plays a crucial role in these laboratories and cleanrooms. That’s why QbD developed QbD Module – an Electronic Batch Recording (EBR) system specifically designed for ATMPs.
Although the workflow around ATMPs is getting more and more complex, many laboratories still use rudimentary tools, like spreadsheets, MS Word and email, to manage the complex workflows during their manufacturing and quality control processes. Often, this results in many practical problems. Employees miss out on e-mails, have problems recalling earlier communication, use outdated document versions or have problems keeping an overview of documents for review or approval. The result? A significant operational impact. You need extra work to keep managing the paper-based system, you need to investigate errors during batch processing due to wrong procedures and – if worst comes to worst – whole batches must be scrapped.
Meet QbD Module : the EBR system for ATMPs
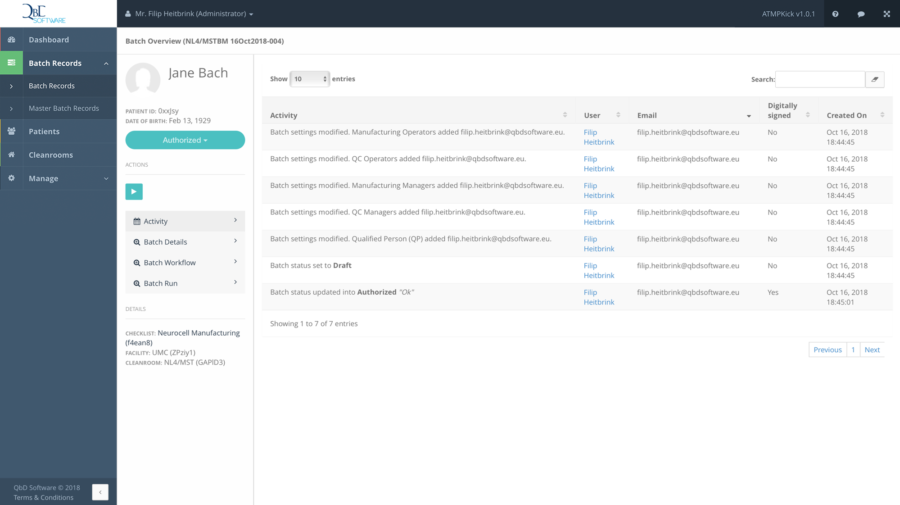
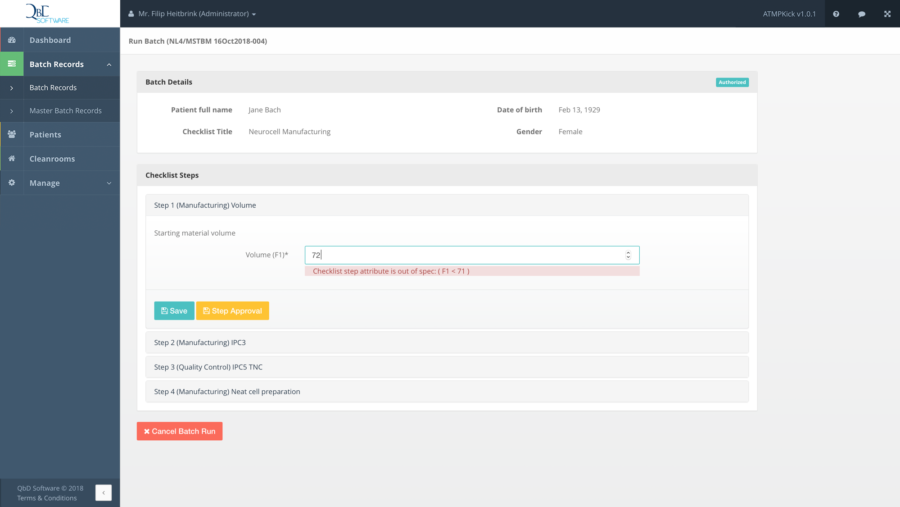
QbD Module is a web-based software solution specifically designed for ATMPs. It enables you to manage production workflows and quality control during the ATMP manufacturing process, and to stepwise run batches under regulatory rules. The system’s core features include the management of patients, facilities, facility locations and cleanrooms. In addition, users are able to create, manage and validate their own specific checklists – including audit trail – thanks to a user-friendly point-and-click interface.
Benefits of QbD Module
An easily accessible web-based solution
Specifically designed for ATMPs, allowing centralized follow-up and release of multiple, decentralised production sites
Customisability for different types of manufacturing sites
Suitable for decentralised and personalised manufacturing
Possibilities for dynamic checklist creation and batch runs
Developed according to FDA 21 CFR part 11 guidelines
Cloud-based set-up with backup and disaster recovery out-of-the-box
Assignment of different roles and tasks to different people
Possibilities for integration with your own systems
Very easy to validate, as validation was a key factor in its development
With QbD Module, you easily digitise and automate ATMP workflows and are able to work remotely with multiple cleanrooms, hospitals and operators. Other advantages include:
How we can support you
It’s our mission to build the right software applications and user interfaces to support companies in the pharmaceutical, biotechnology and life sciences industries in their daily activities. We help you to perform complex tasks in a more user-friendly, accurate and faster way. Our software portfolio includes Scilife™ QMS, a quality management system in the cloud, and various custom-made validated software solutions.
Contact
Request a demo
Receive detailed information from one of our medical device experts.