Master your ATMP development process
with the QbD Module
ATMPS ARE REVOLUTIONIZING HEALTHCARE
Advanced Therapy Medicinal Products (ATMP) are a reality. They are revolutionizing healthcare by introducing highly effective treatments for various diseases and injuries.
The QbD Module helps you resolve the abundant challenges currently faced in the development and production of a new ATMP.
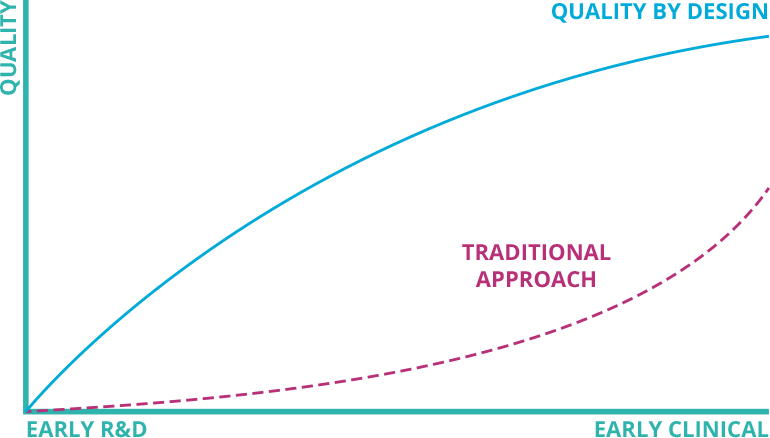
Put quality first in your process development roadmap
QbD Module enables you to opt for a quality-focused ATMP development process by executing risk-based process assessments.
The insights guide science and data driven decisions to meet all predefined critical quality attributes of the ATMPs.
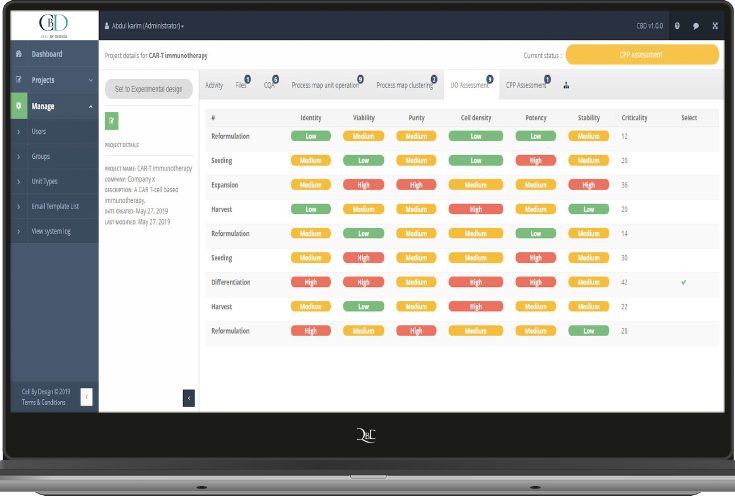
Ensure a closed and automated ATMP development process
Your ATMP success also depends on the manufacturability and scalability of your developed product and process.
Therefore, the QbD Module takes these into account right from the start, to ensure a 100% closed and automated process. Based on the design space built by QbD and Scilife you can apply more affordable parametric product releases.
Process quality
Manufacturability
Scalability
Gear up with the cloud-based QbD module software platform
You will find the cloud-based QbD Module software platform very intuitive. Compatible with Industry 4.0 standards, QbD Module facilitates process automation and the use of machine learning methods and predictive modelling.
Make maximum use of the extensive process expertise contained within the QbD Module. Follow a structured approach in developing new ATMPs which facilitate market authorization and introduction.
TARGET
Define end product specifications and critical attributes
MAP AND RISK ASSESSMENT
Describe unit operations and identify risks for prioritization efforts
PROVE
Gather and analyze data through efficient experiment campaigns designed on the Risk Assessment outcome
CONTROL
Reduce traditional QC by applying novel technologies
Service tailored to your specific needs
The application of QbD Module can be tailored to your specific needs. It can range from a close collaboration (in which you have full access to the software and can count on specialists’ advice in process development and in regulatory challenges) to any particular support you need for your specific ATMP product to process.
INTERESTED IN MAXIMIZING YOUR ATMP SUCCESS?
Contact
Request a demo
Receive detailed information from one of our ATMP experts.