Advanced Therapy Medicinal Products
Are you an advanced therapy medicinal products (ATMP) developer looking to overcome the complexities of ATMPs and navigate a pathway to approval?
The QbD Group can offer your company comprehensive support and solutions across the development, registration, and commercialization of ATMPs.
Our dedicated team of experts from regulatory affairs, quality assurance, QMS establishment, and process development functions can offer advice and solutions to the specific challenges faced.
Discover more on our ATMP expertise below.

Challenges in ATMP
The ATMP industry is a fast-growing field, which has gained high momentum in the last decade with over 20 products granted initial approval in the EU or US.
Current projections forecast a market growth from USD 18 billion in 2022 to USD 82 billion in 2032, with a predicted annual growth rate of >15%.
Nevertheless, there are still several challenges associated with ATMPs and room for improvement. Demonstrating that ATMPs provide value for money to healthcare providers can be problematic due to the high cost of these therapies.
This can be attributed to a number of factors: the high regulatory requirements and development costs, combined with complex manufacturing processes resulting from handling living tissues or generating GMOs.
Major efforts are being devoted to tackle these challenges, in part driven by the remarkable efficacy observed with several ATMPs.

Why QbD?
The ATMP core team within the QbD Group consists of highly skilled professionals in the different aspects and steps of the ATMP product lifecycle.
Our experts in process development, manufacturing, quality control, regulatory affairs and quality management can help you successfully develop and register your ATMP.
Thanks to our Scilife software, we provide support in following the Quality by Design method to develop and/or optimise your manufacturing processes.

Regulatory Affairs Support
Strategic advice
QbD Group can offer strategic advice and support important regulatory interactions to achieve your product development objectives.
Initial Classification & Development Phase
Early in the product lifecycle, this can include submission of a classification application to the Committee for Advanced Therapies (CAT), part of the European Medicines Agency (EMA). CAT will determine if your product can be classified under one of the following ATMP categories defined in Article 17 of Regulation (EC) No 1394/2007:
- Tissue engineered product
- Somatic cell therapy medicinal product
- Gene therapy medicinal product
- Combined ATMP
ATMPs are often developed in areas of high unmet medical need and our team has a wealth of experience working with products developed for the treatment of orphan and rare diseases.
Early Regulatory Interactions
As such we can support you through other early regulatory interactions such as:
- Scientific Advice via EMA or a National Competent Authority
- Submission of an application for Orphan Designation
- Development of a Paediatric Investigation Plan (PIP)
- Advise on application to EMA’s PRIority MEdicines (PRIME) scheme or MHRA’s Innovative Licensing and Access Pathway (ILAP)
Clinical Trial Phase
When you are ready to progress to the clinic, we can also support the preparation and submission of an application for a clinical trial authorization (CTA) in the EU or UK.
Our regulatory experts can support the preparation, technical review and submission of your CTA for assessment under the new EU Clinical Trials Regulation (Regulation (EU) No 536/2014) or the UK’s Combined Review process.
SME & Academic Support
QbD Group can also advise on other incentives that may apply; particularly if you are an academic institution, or your company qualifies as a micro, small and medium-sized enterprise (SME status). Development of ATMPs can pose challenges, especially in CMC aspects and the design of non-clinical studies where standard experimental models may not be applicable.
- EMA offers SMEs a certification process that examines the CMC or non-clinical data generated to date, with the aim of identifying any potential issues ahead of submission of a marketing authorisation application (MAA).
Marketing Authorisation Application (MAA) Submission
We can offer full Project Management and Regulatory Operations support to enable successful preparation and submission of an MAA in eCTD format for assessment by EMA or MHRA.
- Our experience gained with applications reviewed by EMA under both standard and accelerated assessment will help prioritise your resources to facilitate responding to questions efficiently during the procedure.
Post-Approval Guidance
Post-approval, QbD Group are familiar with the additional regulatory requirements associated with products authorised in the EU or UK under exceptional circumstances or with a conditional marketing authorisation and you will benefit from our experience and support to navigate these aspects successfully.
Scilife Software
Furthermore, thanks to our Scilife software, we provide support in following the Quality by Design method to develop and/or optimize your process.
This module, combined with our expertise, leads you through thorough risk analysis, a pragmatic approach in designing the required experiments, scaling out/up, and eventually manage the process costs by the means of a cost of good analysis.
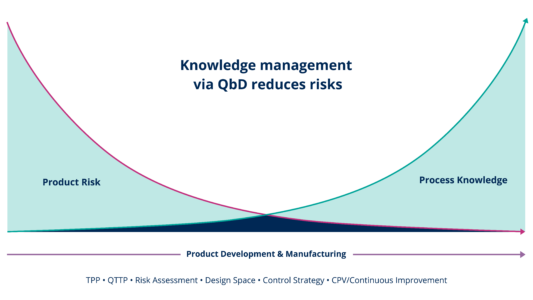
The Quality by Design method is a methodology and a quality system to manage the product’s lifecycle. It is a multifunctional exercise, intended to increase process and product understanding and thereby decrease patient risk. The Quality by Design methodology is woven into the regulatory Guidance Documents (FDA, ICH).
The two critical starting points of the Quality by Design methodology are knowledge and risk. The decisions taken are based on the knowledge of the product and the process, followed by the risk assessment including all the key elements of the process: critical Quality Attributes (cQAs), critical Process Parameters (cPPs) and critical Material Attributes (cMAs).
This systematic approach to process developments will lead to the design of a capable process, which can be implemented at any stage of the product’s life cycle. It will encourage continuous improvements and the risk assessment will be the base of the continuous process verification (CPV).
The QbD module, included in Scilife, is designed to take the user “by hand” through the application of the Quality by Design methodology: the definition of cQAs, cPPs and cMAs, mapping the process, and performing a double risk assessment to define a pragmatic process development approach to drive the development efforts toward the most critical parameters emerged from the risk assessment.
Master your ATMP development process
The QbD Module helps you resolve the abundant challenges currently faced in the development and production of a new ATMP. It enables you to opt for a quality-focused ATMP development process by executing risk-based process assessments.
The application of QbD Module can be tailored to your specific needs. It can range from a close collaboration (in which you have full access to the software and can count on specialists’ advice in process development and in regulatory challenges) to any particular support you need for your specific ATMP product to process.
Contact us
Don’t hesitate to contact us so we can listen to your needs and provide you with the right services.